
While the rest of the world seems to move towards a dead level of uniformity, the living organism is evolving new substances and more and more intricate forms. These processes tear down, living things build up. Lewis 2 referred to living organisms as “cheats in the game of entropy”, which “alone seem able to breast the great stream of apparently irreversible processes. Since then, opinions on both sides have been expressed, although a majority would probably be found in favour of the view that any local increase of ‘free’ energy is compensated by a greater amount of dissipation elsewhere, or as Schrödinger has recently put it 1 in picturesque if somewhat inaccurate language, the organism feeds on ‘negative entropy’. Kelvin left the matter open in his formulation of the Second Law, by expressly excluding the operations of ‘animate agencies’. WHETHER life processes obey the second law of thermodynamics or if life finds a way of evading the otherwise universal dissipation of energy has been something of a puzzle for a century. This page is licensed under this Creative Commons License. The potential of a system toĭo work is determined quantitatively by itsĬontent of this page last modified: 201014 It is clear from the Second Law that not all energy can be used to do work. Kelvin-Planck and Clausius statements are equivalent.

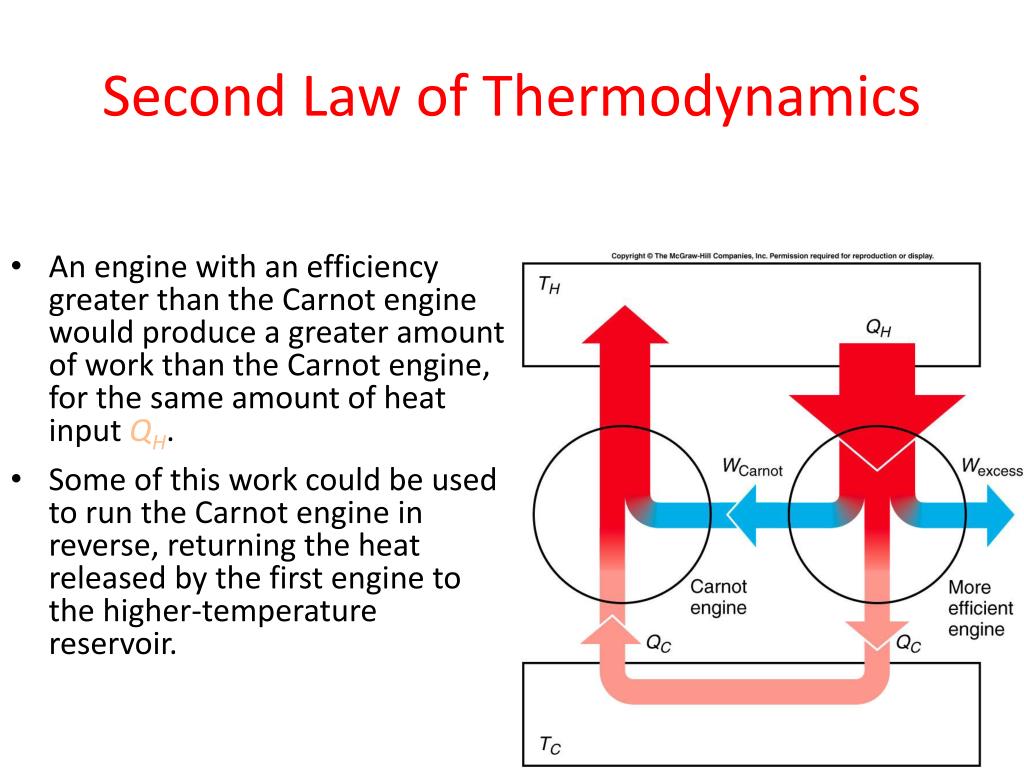
In a very similar argument, it can be shown that a Clausius-violating heat pump coupled with an ordinary heat engine $$Q_H''=Q_L''=Q_L'\qquad.$$ Therefore, the net effect of combining a Kelvin-Planck violating heat engine withĪ heat pump is a Clausius-violating heat pump. The net amount of heat exchanged with the hot reservoir is the same as the heat taken from the cool reservoir Transferred is equal to the heat taken out of the hot reservoir Generated by this unfeasible heat engine to feed the heat pump, heat will flow from cold to hot. We can show that the Clausius statement and the Kelvin-Planck statement amount to the same thing.Ĭonsider a heat engine that violates the Kelvin-Planck statement and an ordinary heat pump. Microscopic processes involved in the operation of the refrigerator: no velocity vectors need re-orientating. While this is quite intuitive, it is not as immediately obvious as the Kelvin-Planck statement when considering the The Second Law according to Kelvin and Planck This is the work done divided by the heat transferred from the fuel: Of any process is, generally speaking, the ratio of desired outputs to required inputs. Here the gas is transformedīack into a liquid which is then pumped back into the boiler to complete the cycle. through a cooling tower or nearby river). ToĪvoid this, the gas is fed through a condenser, a section of pipe with diathermal walls where heat can pass It would short-circuit the cycle, effectively reducing the pressure on the upstream side of the turbine. The turbine effectively acts as aĪfter the turbine, the pressure of the steam will have reduced, but it cannot be fed back into the boiler as This is the point at which ordered energy -work- is taken from the system. The turbine selects the velocity components of the steam atoms which happen to point towards the turbine blades. The second law accounts for the fact that a heat engine can never be completely efficient that is, it cannot convert all the heat energy from its fuel. For example, according to this law, heat will, of its own accord, flows only from hot to a colder object.
#Second law of thermodynamics example generator
The pressurised steamĭrives a turbine, producing work, which can be used to drive a generator producing electricity. The second law of thermodynamics deals with the natural direction of energy processes. liquid water is turned into high-pressure steam. Heat coming from a combustion or nuclear reaction is fed into the circuit in a boiler, where Conventional power stations typically have a circuit in which water is

Energy itself is not a vector quantity, therefore there is no quantitativeĭifference between both forms of energy, but they have a different On the other hand, if all the velocity vectors are oriented in the sameĭirection, the motion of individual atoms adds up to a macroscopic movement, and momentum is transferredįorm of energy is work. Velocity (or momentum) vectors of all atoms cancel out, and no net momentum is observed.
If these movements occur in random directions, the individual For example, if we look at the piston in an engine, the gas is heated to increase its pressure and drive a piston. The second law clearly explains that it is impossible to convert heat energy to mechanical energy with 100 per cent efficiency. Of the atoms or molecules of the system involved in the process. The second law is also known as the Law of Increased Entropy. Processes, heat and work, is an interpretation in terms of the Is an engine that takes in heat and transforms some of it into work.Ī useful way to consider the difference between the two forms of energy transferred during thermodynamic Similarly, heat transfers can be used to let Is a cyclic process which uses work to transfer heat. Thermodynamics Heat engines and the Second Law


 0 kommentar(er)
0 kommentar(er)
